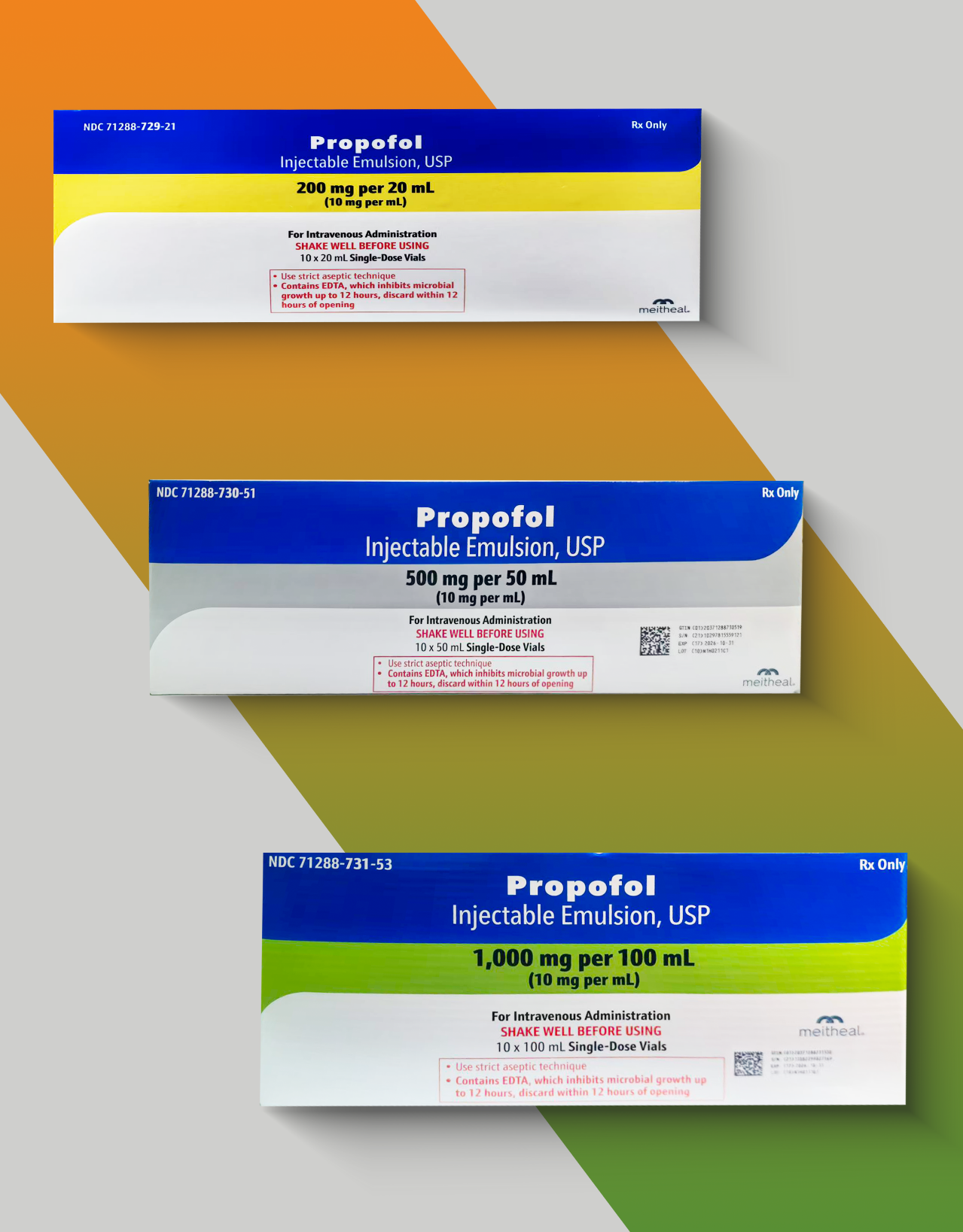
Recently, Nanjing King-Friend Biochemical Pharmaceutical Co.,Ltd. (hereinafter referred to as "NKF") independently developed Propofol Injectable Emulsion has officially received ANDA approval from the US Food and Drug Administration (FDA) (Approval Number: 217945), with specifications covering 200mg/20mL, 500mg/50ml and 1000mg/100mL, and is suitable for general anesthesia and sedation treatment.
The internationalization process has gained another solid weight, highlighting global competitiveness.
The achievement of this important milestone means that we have taken another solid step in the internationalization process of high-end preparations and further consolidated NKF 's product layout and supply capacity in the US anesthetic and sedative drug market.
The approval of this product is an important achievement of our comprehensive expansion into the global market. It is a full recognition of our R&D strength, quality system and international registration capabilities, and reflects the company's continuous efforts to provide high-quality drugs for patients around the world.
Continuous investment and teamwork lead to success
For many years, we have adhered to the pure and devout spirit of pharmaceutical professionals, and have always been committed to fulfilling our mission and responsibility of "driving the development of domestic drugs with international quality, entering the international drug market with international capabilities, and striving for the health of society and humanity." NKF has cumulatively invested approximately 73.66 million yuan in research and development funds for the propofol project, continuously making breakthroughs and striving for excellence. This approval is not only a technological victory, but also the result of teamwork and perseverance.
The future is promising: performance growth and the globalization strategy are steadily advancing
The product is about to be launched in the United States and is expected to bring positive performance contributions to the NKF. In the future, we will continue to uphold the vision of "building a world-class biopharmaceutical enterprise", steadily advance our internationalization strategy, and bring more outstanding drugs made in China to the world.






